Glossary - Mm
Browse alphabetically: A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
Magnetic quantum number | (m Quantum number that labels different orbitals within a subshell. m |
| Main group elements | Elements of the s and p blocks. |
| Malleable | malleability. Capable of being hammered into sheets. Metals are typically malleable materials. |
| Manometer | An instrument for measuring gas pressures. A mercury or oil manometer measures gas pressure as the height of a fluid column the gas sample is able to support. Open manometers measure gas pressure relative to atmospheric pressure. |
| Mass number | (M,A) The total number of protons and neutrons in an atom or ion. In nuclide symbols the mass number is given as a leading superscript. In isotope names (e. g. carbon-14, sodium-23) the mass number is the number following the element name. |
| Mass percentage | ((w/w)%) Mass percentages express the concentration of a component in a mixture or an element in a compound. For example, household bleach is 5.25% NaOCl by mass, meaning that every 100 g of bleach contains 5.25 g of NaOCl. Mass percentage can be calculated as 100% times the mass of a component divided by the mass of the mixture containing the component. |
| Mass spectrometer | An instrument that measures the masses and relative abundances of a sample that has been vaporized and ionized.
|
| Mass spectrometry | mass spectroscopy. (of elements) A method for experimentally determining isotopic masses and isotopic abundances. A sample of an element is converted into a stream of ions and passed through an electromagnetic field. Ions with different charge-to-mass ratios are deflected by different amounts, and strike different spots on a film plate or other detector. From the position of the spots, the mass of the ions can be determined; from the intensity of the spot, the relative number of ions (the isotopic abundance) can be determined. |
| Mass spectrum | mass spectra. A plot showing the results of a mass spectrometry experiment, which shows the presence of particles with different masses as a series of sharp, separate peaks. The position of the peaks on the x-axis indicates the mass of the particles; the peak heights indicate the relative abundance of the particles. |
| Mass | (m) Mass is a measure of the tendency of an object to resist acceleration. It's harder to roll a tractor trailer than a roller skate; the tractor trailer has a far greater mass. |
| Matter | Matter is anything that has mass. Air, water, coffee, fire, human beings, and stars are matter. Light, X-rays, photons, gravitons, information, and love aren't matter. |
| Measurement | Measurement is the collection of quantitative data. Measurement involves comparison of the quantity of interest with a standard called a unit. The comparison is never perfect. As a result, measurements always include error. You must consider the reliability of the measurement when using it to make decisions or estimate other quantities. |
| Medicinal chemistry | A branch of chemistry concerned with the discovery, design, synthesis, and investigation of biologically active compounds and reactions that these compounds undergo in living things. |
| Mega- | (M) mega. SI prefix meaning "multiply by 106". For example, 3.2 MJ is 3200000 J. |
| Meniscus | meniscuses; menisci. A phase boundary that is curved because of surface tension. |
| Metabolism | metabolic; metabolic reaction. A sequence of biochemical reactions that converts fuel molecules into energy used to drive other biological processes. Also refers to the sequence of transformations foreign compounds undergo inside a living cell. |
| Metabolite | A compound produced by metabolic reactions. |
| Metal | metallic. A metal is a substance that conducts heat and electricity, is shiny and reflects many colours of light, and can be hammered into sheets or drawn into wire. Metals lose electrons easily to form cations. About 80% of the known chemical elements are metals. |
| Metallic compounds | Compounds that contain at least one metallic element. |
| Metalloid | semimetal; semi-metal. An element with both metallic and nonmetallic properties. Examples are silicon, arsenic, and germanium. |
| Metre | (m) The meter is the basic unit of length in the SI system of units, defined as the distance light travels through a vacuum in exactly 1/299792458 seconds. 1 m = 39.37 inches. Meters are abbreviated as "m" in measurements. |
| Methionine | 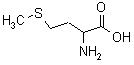 A naturally occurring amino acid and building block of proteins with a sulfur-containing side chain. |
| Methyl | (-CH3) A group -CH3, derived from methane. For example, CH3Cl is "methyl chloride" (systematic name: chloromethane); CH3OH is "methyl alcohol" (systematic name: methanol). |
| Micron | (µm) micrometre. A unit of length, equivalent to 10-6 meters. |
| Microwave | microwave radiation. Electromagnetic radiation with wavelength between 3 mm and 30 cm. |
| Micro- | (µ) Prefix used in the SI system meaning "one millionth of". For example 1 µm means "one millionth of a meter"; 3.1 µL means "3.1 × 10-6 L". |
| milli- | (m) Prefix used in the SI system meaning "one thousandth of". For example 1 mL means "one thousandth of a litre"; 1 mg means "one thousandth of a gram". |
| Miscible | miscibility; liquid miscibility. Two liquids are considered "miscible" or mixable if shaking them together results in a single liquid phase, with no meniscus visible between layers of liquid. |
| Mistake | A mistake is a measurement which is known to be incorrect due to carelessness, accidents, or the ineptitude of the experimenter. It's important to distinguish mistakes from errors: mistakes can be avoided. Errors can be minimized but not entirely avoided, because they are part of the process of measurement. Data that is mistaken should be discarded. Data that contains errors can be useful, if the sizes of the errors can be estimated. |
| Mixed glyceride | A diglyceride or triglyceride that contains more than one type of fatty acid connected to glycerol via an ester linkage. Natural oils and fats usually contain several different mixed glycerides. |
| Molality | (m) Concentration measured as moles of solute per kilogram of solvent. For example, a 1 m NaCl solution contains 1 mole of NaCl per kilogram of water. Molalities are preferred over molarities in experiments that involve temperature changes of solutions, e. g. calorimetry and freezing point depression experiments. |
| Molar | 1. Of or pertaining to moles. 2. An synonym for molarity; for example, a "six molar solution of hydrochloric acid" contains 6 moles of HCl per litre of solution. |
| Molar absorptivity | ( The absorbance per centimetre of path length when the concentration of absorbing material is 1 M; |
| Molar heat capacity | atomic heat capacity. The heat required to raise the temperature of one mole of a substance by 1°C is called the molar heat capacity of the substance. Molar heat capacity is an intensive property with SI system units of J mol-1 K-1. The molar heat capacity of elements is sometimes called the "atomic heat capacity". |
| Molar mass | The mass of one mole of a material. For example, the molar mass of H2O is 18.015 g (obtained by adding twice the molar mass of hydrogen to the molar mass of oxygen). |
| Molar volume | The volume occupied by one mole of a material. For example, the molar volume of an ideal gas at STP is 22.4 L/mol. |
| Molarity | (M) molar concentration. Concentration of a solution measured as the number of moles of solute per litre of solution. For example, a 6 M HCl solution contains 6 moles of HCl per liter of solution. |
| Mole | (mol) SI unit for amount of substance, defined as the number of atoms in exactly 12 g of carbon-12. One mole of a molecular compound contains Avogadro's number molecules and has a mass equal to the substance's molecular weight, in grams. |
| Mole fraction | Concentration of a substance in a mixture measured as moles of the substance per mole of mixture. For example, the mole fraction of oxygen in air is about 0.21, which means that 1 mol of air contains about 0.21 mol O2. |
| Molecular equation | A molecular equation is a balanced chemical equation in which ionic compounds are written as neutral formulas rather than as ions. For example, AgNO3(aq) + NaCl(aq) = AgCl(s) + NaNO3(aq) is a molecular equation; Ag+(aq) + NO3-(aq) + Na+(aq) + Cl-(aq) = AgCl(s) + Na+(aq) + NO3-(aq) is not. |
| Molecular formula | A notation that indicates the type and number of atoms in a molecule. The molecular formula of glucose is C6H12O6, which indicates that a molecule of glucose contains 6 atoms of carbon, 12 atoms of hydrogen, and 6 atoms of oxygen. |
| Molecular geometry | 1. The three-dimensional shape of a molecule. For example, methane (CH4) has a tetrahedral molecular geometry. 2. The study of molecular shapes. |
| Molecular model | stick model; ball and stick model; space filling model. A representation of a molecule. The model can be purely computational or it can be an actual physical object. Stick models show bonds, ball-and-stick models show bonds and atoms, and space filling models show relative atomic sizes. |
| Molecular orbital | A wavefunction that describes the behaviour of an electron in a molecule. Molecular orbitals are usually spread across many atoms in the molecule, and they are often described as a combination of atomic orbitals on those atoms. |
| Molecular sieve | A material that contains many small cavities interconnected with pores of precisely uniform size. Zeolites are an example. Molecular sieves adsorb molecules that are small enough to pass through their pore systems- especially water. They are often used as drying agents, and to separate large molecules from smaller ones in preparatory work and in exclusion chromatography. |
| Molecular weight | molecular mass. The average mass of a molecule, calculated by summing the atomic weights of atoms in the molecular formula. Note that the words mass and weight are often used interchangeably in chemistry. |
| Molecule | The smallest particle of an element or compound that retains the chemical properties of the element or compound. A molecule is a collection of chemically bound atoms with characteristic composition and structure. Making or breaking bonds in a molecule changes it into a new molecule. Ionic compounds are not composed of molecules, because there is no distinct collection of ions that are chemically bound in the crystal. |
| Momentum | (p) Momentum is a property that measures the tendency of a moving object to keep moving in the same direction. Increasing the speed of an object increases its momentum, and a heavy object will have more momentum than a lighter one moving at the same speed. For a particle with mass m and velocity v, the momentum of the particle is mv. |
| Monochromatic | Radiation that has a single wavelength. |
| Monodentate | A ligand that has only one atom that coordinates directly to the central atom in a complex. For example, ammonia and chloride ion are monodentate ligands of copper in the complexes [Cu(NH3)6]2+ and [CuCl6]2+. |
| Monomer | A small molecule that is linked with large numbers of other small molecules to form a chain or a network (polymer). |
| Monosaccharide | simple sugar. A carbohydrate that cannot be decomposed into simpler carbohydrates by hydrolysis. |
| Mother liquor | The solution in recrystallization. |
| MSDS | material safety data sheet. Safety information sheet for a particular substance that lists physical properties, hazards, cleanup and disposal procedures, fire and explosion data, and protective equipment required. |
| MSG | monosodium glutamate. MSG is monosodium glutamate, used as a flavour enhancer in many foods. |
| Multiple bond | Sharing of more than one electron pair between bonded atoms. A double bond consists of two shared pairs of electrons; a triple bond consists of three shared pairs. |
Document Actions

 Like us on Facebook
Like us on Facebook