Glossary - Ff
Browse alphabetically: A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
f orbital | f-orbital. An orbital with angular momentum quantum number |
| Fahrenheit | (°F) A temperature scale proposed by Daniel Gabriel Fahrenheit (1686-1736) which uses the melting point of ice (32°F) and the boiling point of water at one atmosphere (212°F) as calibration points. |
| Fatty acid | Fatty acids are carboxylic acids with long hydrocarbon side chains. Most natural fatty acids have hydrocarbon chains that don't branch; any double bonds occurring in the chain are cis isomers (side chains are attached on the same side of the double bond).
|
| Femto- | (f) Prefix used in the SI system meaning "multiply by 10-15". For example 22 fg means 22× 10-15 g. |
| Fermentation | A class of biochemical reactions that break down complex organic molecules (such as carbohydrates) into simpler materials (such as ethanol, carbon dioxide, and water). Fermentation reactions are catalysed by enzymes. |
| Ferroin | A blood-red complex of Fe2+ ion with 1,10-phenanthroline, used as a redox indicator. Ferroin changes from red to pale blue when oxidized. |
| Ferromagnetism | ferromagnetic Ferromagnetic materials exhibit magnetism even in the absence of an external magnetic field. Ferromagnetic materials contain regions where many paramagnetic atoms or ions have magnetic moments that are aligned in the same direction. Iron, cobalt, nickel, and gadolinium are elements that can exhibit ferromagnetic behaviour. |
| Ferric | ferric ion. Deprecated. 1. the iron(III) ion, Fe3+. 2. A compound that contains iron in the +3 oxidation state. |
| Ferrous | ferrous ion. Deprecated. 1. the iron(II) ion, Fe2+. 2. A compound that contains iron in the +2 oxidation state. |
| First ionization energy | (IE,IP) first ionization potential. The energy needed to remove an electron from an isolated, neutral atom. |
| First law | first law of thermodynamics. The first law states that energy cannot be created or destroyed. Many equivalent statements are possible, including: Internal energy changes depend only on the initial and final states of the system, not on the path taken. The work done during an adiabatic process depends only on the initial and final states of the system, and not on the path taken. The internal energy change for any cyclic process is zero. |
| First order reaction | The sum of concentration exponents in the rate law for a first order reaction is one. Many radioactive decays are first order reactions. |
| Fission | nuclear fission. A nuclear reaction in which an atomic nucleus breaks into smaller nuclei of comparable mass, releasing a large amount of energy. |
| Flash point | The temperature when vapour pressure of a substance becomes high enough to allow the air/vapour layer over the substance to be ignited. Ether and acetone have flash points below room temperature, which makes them very dangerous. |
| Fluorescence | fluorescent; fluorescent compound. A fluorescent substance absorbs short wavelength radiation and re-emits it as radiation with a longer wavelength in a very short time. |
| Foam | A colloid in which bubbles of gas are suspended in a solid or liquid. Aerogel (solid smoke) and Styrafoam are examples of solid foams; whipped cream is an example of a liquid foam. |
| Formation | formation reaction. A reaction that forms one mole of a compound from its elements in their most stable forms. For example, the formation reaction for water is H2(g) + ½O2 |
| Formula weight | formula mass. The formula weight is the sum of the atomic weights of the atoms in an empirical formula. Formula weights are usually written in atomic mass units (u). |
| Formula unit | One formula weight of a compound. |
| Fractional distillation | A technique for separation of liquid mixtures by distillation that uses a tower attached to a flask containing the mixture to perform multiple distillations. Vapour moving up the column condenses on packing material inside the column, trickles down the column, and again vaporises. The more volatile component can then be drawn off at the top of the component, while the less volatile component remains at the bottom. |
| Free energy | Energy that is actually available to do useful work. A decrease in free energy accompanies any spontaneous process. Free energy does not change for systems that are at equilibrium. |
| Free radical | A free radical is a molecule with an odd number of electrons. Free radicals do not have a completed octet and often undergo vigorous redox reactions. Free radicals produced within cells can react with membranes, enzymes, and genetic material, damaging or even killing the cell. Free radicals have been implicated in a number of degenerative conditions, from natural aging to Alzheimer's disease. |
| Freezing point | The temperature at which the vapour pressure of a liquid is equal to the vapour pressure of the corresponding solid form. The liquid and solid forms can coexist at equilibrium at the freezing point. The standard melting point is the melting point at standard pressure. |
| Freezing point depression | ( The freezing point of a solution is always lower than the freezing point of the pure solvent. The freezing point depression is roughly proportional to the molality of solute particles in the solution. Freezing point depression is an example of a colligative property of a solution. |
| Frequency | ( The number of cycles of a wave that move past a fixed observation point per second. The SI unit of frequency is the Hertz (Hz). |
| Fuel cell | A device that converts the chemical energy obtained from a redox reaction directly into electrical energy. |
| Functional group | A substructure that imparts characteristic chemical behaviours to a molecule, for example, a carboxylic acid group. |
Document Actions

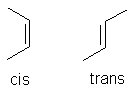
 Like us on Facebook
Like us on Facebook