Glossary - Ll
Browse alphabetically: A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
L- | L-isomer. Prefix used to designate a levorotatory enantiomer. |
| Lambert's law | The intensity of radiation passing through a material decays exponentially with path length b. |
| Lanthanide contraction | An effect that causes sixth period elements with filled 4f subshells to be smaller than otherwise expected. The intervention of the lanthanides increases the effective nuclear charge, which offsets the size increase expected from filling the n=6 valence shell. As a consequence, sixth period transition metals are about the same size as their fifth period counterparts. |
| Lanthanide | Elements 57-70 are called lanthanides. Electrons added during the Aufbau construction of lanthanide atoms go into the 4f subshell. |
| Latent heat | Heat that is absorbed without causing a rise in temperature. For example, "latent heat of vaporization" refers to the amount of heat required to convert a liquid to vapour at a particular temperature. |
| Lattice | A regular array of ions or atoms. |
| Law of combining volumes | Gay-Lussac's law. When gases react, they do so in a definite proportion by volume, if the volumes are measured at the same pressure and temperature. For example, in the reaction N2(g) + 3 H2(g) = 2 NH3(g), 3 litres of hydrogen will react with 1 litre of nitrogen to give 2 litres of ammonia if all volumes are measured at the same temperature and pressure. |
| Law of conservation of mass | There is no change in total mass during a chemical change. The demonstration of conservation of mass by Antoine Lavoisier in the late 18th century was a milestone in the development of modern chemistry. |
| Law of definite proportions | When two pure substances react to form a compound, they do so in a definite proportion by mass. For example, when water is formed from the reaction between hydrogen and oxygen, the 'definite proportion' is 1 g of H for every 8 g of O. |
| Law of multiple proportions | When one element can combine with another to form more than one compound, the mass ratios of the elements in the compounds are simple whole-number ratios of each other. For example, in CO and in CO2, the oxygen-to-carbon ratios are 16:12 and 32:12, respectively. Note that the second ratio is exactly twice the first, because there are exactly twice as many oxygens in CO2 per carbon as there are in CO. |
| Law | natural law; scientific law. Natural laws summarize patterns that recur in a large amount of data. Unlike human laws, natural laws don't forbid or permit; they describe. |
| Le Chatelier's principle | Le Chatelier's principle predicts that when a stress is applied to an equilibrium mixture, the equilibrium will shift to relieve the stress. Stresses include temperature changes, pressure changes, and changes in the concentrations of species in the mixture. For example, increasing the concentration of a reactant drives the reaction forward; increasing the concentration of a product drives it backward. |
| Leucine | ((CH3)2CHCH2CH(NH2)COOH) A naturally occuring aliphatic amino acid with a nonpolar side chain. |
| Levorotatory | Having the property of rotating plane-polarized light counter clockwise. |
| Lewis structure | electron dot structure; dot structure. A model pioneered by Gilbert N. Lewis and Irving Langmuir that represents the electronic structure of a molecule by writing the valence electrons of atoms as dots. Pairs of dots (or lines) wedged between atoms represent bonds; dots drawn elsewhere represent nonbonding electrons. |
| Ligand | 1. In inorganic chemistry, a molecule or ion that binds to a metal cation to form a complex. 2. In biochemistry, a molecule that binds to a receptor, having a biological effect. |
| Limit of quantitation | (LOD) quantitative detection limit; limit of determination. The smallest detectable concentration an analytical instrument can determine at a given confidence level. IUPAC defines the quantitative detection limit as Cld = ks/m, where k is 10, s is the standard deviation of instrument readings taken on a "blank" (a solution with zero concentration of analyte), and m is the slope of a plot of instrument response vs. concentration, as calculated by linear regression. |
| Limiting reactant | The reactant that limits the amount of product produced in a chemical reaction. For example, mixing one mole of H2(g) with one mole of O2 produces one mole of steam (H2O(g)), with half a mole of O2(g) remaining. The hydrogen gas limits the amount of steam produced in this case. |
| Line spectrum | A emission spectrum that contains very sharp peaks, corresponding to transitions between states in free atoms. For example, the line spectrum of hydrogen contains 4 sharp lines in the visible part of the spectrum. |
| Lipid | A diverse group of organic molecules that contain long hydrocarbon chains or rings and are hydrophobic. Examples are fats, oils, waxes, and steroids. |
| Lipophilic | lipophilicity. Refers to a substance's solubility in fat. Lipophilicity can be measured by shaking the substance with a two-phase mixture of water and 1-octanol and observing the final concentrations of the substance in the two layers. Lipophilic substances will move into the 1-octanol layer, while hydrophilic substances stay in the water. |
| Liquid | A state of matter that has a high density and is incompressible compared to a gas. Liquids take the shape of their container but do not expand to fill the container as gases do. Liquids diffuse much more slowly than gases. |
| Lithium | (Li) Li. Element 3, atomic weight 6.939. The lightest alkali metal, used in special-purpose metal alloys and other industrial applications. |
| Litmus | A mixture of pigments extracted from certain lichens that turns blue in basic solution and red in acidic solution. |
| Litmus paper | litmus test. Paper impregnated with litmus, usually cut in narrow strips. Dipping red litmus paper into a basic solution turns it blue; dipping blue litmus paper into an acidic solution turns it red. |
| Lock and key model | A model that explains the role of enzymes in chemical reactions by assuming that the reactants fit into the enzyme like a key fits into a lock. |
| London force | dispersion force. An intermolecular attractive force that arises from a cooperative oscillation of electron clouds on a collection of molecules at close range. |
| Lone pair | nonbonding pair; unshared pair. Electrons that are not involved in bonding. |
| Low spin complex | A metal-ligand complex with fewer unpaired electrons than the uncomplexed metal ion. When a strong ligand complexes the metal ion, the crystal field splitting is large and some electrons pair rather than occupying the higher energy d orbitals. |
| Lysine | 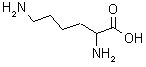 A naturally occurring amino acid with an amine group on its side chain. |
Document Actions

 Like us on Facebook
Like us on Facebook