Glossary - Dd
Browse alphabetically: A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
Dalton's law | Dalton's law of partial pressure. The total pressure exerted by a mixture of gases is the sum of the pressures that each gas would exert if it were alone. For example, if dry oxygen gas at 713 torr is saturated with water vapour at 25 torr, the pressure of the wet gas is 738 torr. |
| Debye | (D) Debye unit. A common non-SI unit of dipole moment, named for Dutch physical chemist Peter Debye. A charge separation equal to one electron charge placed one Ångstrom unit apart has a dipole moment of 4.8 D. In SI units, 1 D = 3.338 × 10-30 coulomb metres. |
| Decomposition | A reaction in which a compound is broken down into simpler compounds or elements. Compounds sometimes decompose if heated strongly or if subjected to a strong electric current (electrolysis). |
| Degenerate | A set of orbitals are said to be degenerate if they all have the same energy. This degeneracy can sometimes be "lifted" by external electric or magnetic fields. |
| Deliquescent | Deliquescent compounds absorb so much moisture from the air that they dissolve. Examples are calcium chloride and sodium hydroxide. |
| Denature | A loss of chemical function, usually due to some heat or chemically-induced structural change. For example, heating a protein causes it to lose its three dimensional form and it no longer functions correctly. |
| Density | ( Mass of a substance per unit volume. Saying "the density of mercury is 13.55 g/cm3 " is the same as saying "the mass of exactly 1 cm3 of mercury is 13.55 g". |
| Density functional | A model that describes the electronic structure of an atom or molecule by approximating the total energy as a function of electron density. |
| Dependent variable | A dependent variable changes in response to changes in independent variables. For example, in an experiment where the vapour pressure of a liquid is measured at several different temperatures, temperature is the independent variable and vapour pressure is the dependent variable. |
| Derived unit | Derived units are units constructed from the system's base units. For example, the SI unit for density is kg/m3, derived from the base units kg and m. |
| Desalination | Removal of dissolved salts from seawater. |
| Destructive interference | When the peaks of one wave match the troughs of another, the waves interfere destructively. The amplitudes of the interfering waves cancel to give the resultant wave lower amplitude. |
| Deuterium | (D, 2H) An isotope of hydrogen that contains one neutron and one proton in its nucleus. |
| Dextrorotatory | Having the property of rotating plane-polarized light clockwise. |
| Dialysis | Dialysis is the separation of components in a mixture by passing them across a semipermeable membrane. |
| Diamagnetism | Diamagnetic materials are very weakly repelled by magnetic fields. The atoms or molecules of diamagnetic materials contain no unpaired spins. |
| Diamond | A crystalline form of carbon, made of a network of covalent, tetrahedrally bound carbon atoms. |
| Diastereomer | Stereoisomers which are not mirror images of each other. Diastereomers are chemically similar but distinguishable; they have different melting points and boiling points and they react at different rates. |
| Diatomic molecule | A molecule that contains only two atoms. All of the noninert gases occur as diatomic molecules; e. g. hydrogen, oxygen, nitrogen, fluorine, and chlorine are H2, O2, N2, F2, and Cl2, respectively. |
| Diazonium salt | A diazonium salt is a compound with general form Ar-N |
| Diazotization | Diazotization is a reaction that converts an -NH2 group connected to a phenyl ring to a diazonium salt. For example,
|
| Dichloromethane | (CH2Cl2) Dichloromethane (CH2Cl2) is an organic solvent often use to extract organic substances from samples. It is toxic but much less so than chloroform or carbon tetrachloride, which were previously used for this purpose. |
| Differential thermal analysis | (DTA) A technique that is often used to analyse materials that react or decompose at higher temperatures. The difference in temperature between the sample and an inert reference material is monitored as both are heated in a furnace. Phase transitions and chemical reactions taking place in the sample on heating cause the temperature difference to become larger, at temperatures that are characteristic of the sample. |
| Diffraction | The ability of a wave to bend around the edges of obstacles or holes. The effect is most noticeable when the obstacle or hole is comparable to the size of the wavelength. |
| Diffusion | The mixing of two substances caused by random molecular motions. Gases diffuse very quickly; liquids diffuse much more slowly, and solids diffuse at very slow (but often measurable) rates. Molecular collisions make diffusion slower in liquids and solids. |
| Diffusion rate | The number of randomly moving molecules that pass through a unit area per second. Diffusion rates are fastest when a large concentration difference exists on either side of the unit area. Diffusion rates increase with temperature, and decrease with increasing pressure, molecular weight, and molecular size. |
| Dilatometer | A device for measuring volume changes. |
| Dilute | Having a relatively low concentration. |
| Dilution | Adding solvent to a solution to lower its concentration. |
| Dipole-dipole interaction | Electrostatic attraction between oppositely charged poles of two or more dipoles. |
| Dissolved oxygen | The amount of oxygen dissolved in a solvent (usually water). Dissolved oxygen levels are used as a general indicator of water quality. |
| Displacement | displacement reaction; replacement reaction; replacement A reaction in which a fragment of one reactant is replaced by another reactant (or by a fragment of another reactant). Displacement reactions have the same number of products as reactants, and are described by equations of the form A + BC |
| Disproportionate | A reaction involving a substance that produces two different forms of the substance, one more oxidized and the other more reduced than the original. |
| Distillate | The vapour collected and condensed from a distillation. |
| Distillation | Distillation is a technique for separating components of a mixture on the basis of differing boiling points. The mixture is heated, vaporizing some of the components. The vapour is collected and condensed to isolate the components with the lowest boiling points. |
| Divalent | Binds to two other things (which may be other atoms, molecules, ions, or electrons). See also divalent anion and divalent cation. |
| Divalent anion | An ion with a charge of -2. |
| Divalent cation | An ion with a charge of +2. |
| DNA | deoxyribonucleic acid A nucleic acid with 2-deoxy-D-ribose as the sugar in its nucleotides. DNA contains encoded genetic information, specifically templates for the synthesis of all of an organism's proteins and enzymes. |
| Domoic acid | Domoic acid is a toxic amino acid produced by certain species of algae. Domoic acid binds to a receptor that helps nerve cells control the flow of ions across their cell membranes. The receptor no longer works correctly, and the uncontrolled flux of ions damages and eventually kills the nerve cell. |
| Double displacement | A double displacement or metathesis is a reaction in which two reactants trade fragments: AB + CD = AC + BD Most commonly, the fragments are ions, e. g. AgNO3(aq) + NaCl(aq) = AgCl(s) + NaNO3(aq) |
| Drug | A biologically active compound or mixture used to cure, prevent, or detect disease, to control biological processes, or to alter mental state. |
| Dry cell | Leclanché cell. A electrolytic cell that uses a moist paste rather than a liquid as an electrolyte. Flashlight batteries are dry cells with a zinc cup for an anode, a carbon rod for a cathode, and a paste made of powdered carbon, NH4Cl, ZnCl2, and MnO2 for an electrolyte. |
| Ductile | Capable of being drawn into wire. Metals are typically ductile materials. |
| Dynamic equilibrium | Dynamic equilibrium is established when two opposing processes are occuring at precisely the same rate, so that there is no apparent change in the system over long periods of time. |
| Dyne | (dyn) The unit of force in the obsolete cgs system of units. A dyne is the force required to accelerate a 1 g mass by 1 cm/s per second. |
Document Actions

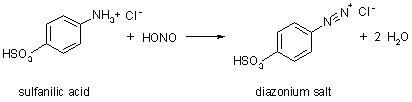
 Like us on Facebook
Like us on Facebook