Glossary - Cc
Browse alphabetically: A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
Caffeine | 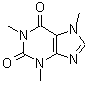 A substance found in tea, coffee, and cola that acts as a stimulant. It is extremely soluble in supercritical fluid carbon dioxide and somewhat soluble in water; aqueous solutions of caffeine quickly break down. |
| Calibration | Calibration is correcting a measuring instrument by measuring values whose true values are known. Calibration minimizes systematic error. |
| Calorie | The amount of heat required to raise the temperature of 1 g of water at 14.5°C to 15.5°C. One calorie is equivalent to exactly 4.184 J. |
| Calorimeter | An insulated vessel for measuring the amount of heat absorbed or released by a chemical or physical change. |
| Calorimetry | Experimental determination of heat absorbed or released by a chemical or physical change. |
| Calutron | A device that separates isotopes (e. g. 235U from 238U) by ionizing the sample, accelerating the ions in a strong electric field, and then passing them through a strong magnetic field. The magnetic field bends the trajectories of the ions with high charge-to-mass ratio more, allowing ions to be separated by mass and collected. |
| Capacitor | A device for storing electric charge, consisting of two metal plates separated by an insulating material. |
| Carbohydrate | carb. A class of organic compounds including sugars and starches. The name comes from the fact that many (but not all) carbohydrates have empirical formula CH2O. |
| Carbon | C. An element with atomic number 6. Carbon is a non-metal found in all organic compounds. Carbon occurs naturally as diamond, graphite, and buckminsterfullerene. |
| Carbon dioxide | (CO_2) A colourless, odourless gas produced by respiration and combustion of carbon-containing fuels. |
| Carbon monoxide | (CO) A colourless, odourless, poisonous gas produced by incomplete combustion. |
| Carbonate | (CO32-) 1. an inorganic ion with a charge of -2, containing carbon bound directly to three oxygen’s in a in a flat triangular arrangement. 2. A compound containing CO32- ions. |
| Carbonate hardness | Water hardness due to the presence of calcium and magnesium carbonates and bicarbonates. The "noncarbonate hardness" is due mostly to calcium and magnesium sulphates, chlorides, and nitrates. |
| Carbonyl | carbonyl group. A divalent group consisting of a carbon atom with a double-bond to oxygen. For example, acetone (CH3-(C=O)-CH3) is a carbonyl group linking two methyl groups. Also refers to a compound of a metal with carbon monoxide, such as iron carbonyl, Fe(CO)5. |
| Carboxylic acid | carboxyl; carboxyl group. A carboxylic acid is an organic molecule with a -(C=O)-OH group. The group is also written as -COOH and is called a carboxyl group. The hydrogen on the -COOH group ionizes in water; carboxylic acids are weak acids. The simplest carboxylic acids are formic acid (H-COOH) and acetic acid (CH3-COOH). |
| Carotene | Carotene is an unsaturated hydrocarbon pigment found in many plants. Carotene is the basic building block of vitamin A. |
| Catalyst | A substance that increases the rate of a chemical reaction, without being consumed or produced by the reaction. Catalysts speed both the forward and reverse reactions, without changing the position of equilibrium. Enzymes are catalysts for many biochemical reactions. |
| Cathode ray | A negatively charged beam that emanates from the cathode of a discharge tube. Cathode rays are streams of electrons. |
| Cathode | The electrode at which reduction occurs. |
| Cation | A cation is a positively charged ion. Metals typically form cations. |
| Cellulose | A polysaccharide made of linked glucose molecules that strengthen the cell walls of most plants. |
| Celsius | (°C) Celsius temperature scale; Celsius scale. A common but non-SI unit of temperature, defined by assigning temperatures of 0°C and 100°C to the freezing and boiling points of water, respectively. |
| centi- | (c) Prefix used in the SI system meaning "one hundredth of". For example 1 cm means "one hundredth of a meter"; 2.3 cg could also be written "2.3 × 10-2 g" or "0.023 g". |
| Charles' law | The volume of a gas is directly proportional to its temperature in kelvins, if pressure and amount of gas remain constant. Doubling the kelvin temperature of a gas at constant pressure will double its volume. If V1 and T1 are the initial volume and temperature, the final volume and temperature ratio V2/T2 = V1/T1 if pressure and moles of gas are unchanged. |
| Chelate | A stable complex of a metal with one or more polydentate ligands. For example, calcium complexes with EDTA to form a chelate. |
| Chelating agent | A ligand that binds to a metal using more than one atom; a polydentate ligand. |
| Chemical | 1 of or pertaining to chemistry. 2. a substance. |
| Chemical bond | A chemical bond is a strong attraction between two or more atoms. Bonds hold atoms in molecules and crystals together. There are many types of chemical bonds, but all involve electrons which are either shared or transferred between the bonded atoms. |
| Chemical change | reaction; chemical reaction. A chemical change is a dissociation, recombination, or rearrangement of atoms. |
| Chemical equation | A compact notation for describing a chemical change. The formulas of the reactants are added together on the left hand side of the equation; the formulas of the products are added together on the right side. Coefficients are inserted before the formulas to ensure that the equation is balanced. The phase in which each substance is found is usually indicated in parentheses after each formula. For example, 2 H2(g) + O2(g) = 2 H2O(g) indicates that 2 moles of hydrogen gas combine with one mole of oxygen gas to produce two moles of steam. |
| Chemical potential | (µ) The chemical potential is a partial molar Gibbs free energy, defined as µi = ( |
| Chemical property | Measurement of a chemical property involves a chemical change. For example, determining the flammability of gasoline involves burning it, producing carbon dioxide and water. |
| Chemiluminescence | A chemical reaction that releases energy as electromagnetic radiation. |
| Chemistry | The study of matter and its transformations |
| Chiral | Having nonsuperimposable mirror images. For example, a shoe or a glove is chiral. |
| Chiral centre | An atom in a molecule that causes chirality, usually an atom that is bound to four different groups. A molecule can have chirality without having a chiral centre, and a molecule may also have more than one chiral centres. |
| Chromatography | Chromatography is a method for separating mixtures based on differences in the speed at which they migrate over or through a stationary phase. |
| Chromophore | A group or substructure on a molecule that is responsible for the absorption of light. |
| Clausius-Clapeyron equation | The Clausius-Clapeyron equation predicts the temperature dependence of vapour pressures of pure liquids or solids:
where P is the vapour pressure, P° is a vapour pressure at a known temperature T°, |
| Cohesion | Attraction between like molecules. |
| Colligative property | Properties of a solution that depend on the number of solute molecules present, but not on the nature of the solute. Osmotic pressure, vapour pressure, freezing point depression, and boiling point elevation are examples of colligative properties. |
| Collision frequency | The average number of collisions that a molecule undergoes each second. |
| Collision theory | A theory that explains reaction rates in terms of collisions between reactant molecules. |
| Colloid | A colloid is a heterogeneous mixture composed of tiny particles suspended in another material. The particles are larger than molecules but less than 1 µm in diameter. Particles this small do not settle out and pass right through filter paper. Milk is an example of a colloid. The particles can be solid, tiny droplets of liquid, or tiny bubbles of gas; the suspending medium can be a solid, liquid, or gas (although gas-gas colloids aren't possible). |
| Colorimetry | A method for chemical analysis that relates colour intensity to the concentration of analyte. |
| Column chromatography | Column chromatography is a method for separating mixtures. A solution containing the mixture is passed through a narrow tube packed with a stationary phase. Different substances in the mixture have different affinities for the stationary phase, and so move through the tube at different rates. This allows the substances in the mixture to be detected or collected separately as they reach the end of the tube. |
| Combination reaction | A reaction in which two or more substances are chemically bonded together to produce a product. For example, 2 Na(s) + Cl2(g) |
| Combustion | combustion reaction. A chemical reaction between a fuel and an oxidizing agent that produces heat (and usually, light). For example, the combustion of methane is represented as CH4(g) + 2 O2(g) = CO2(g) + 2 H2O( |
| Complete combustion | A combustion reaction that converts all of the fuel's carbon, hydrogen, sulphur, and nitrogen into carbon dioxide, water, sulphur dioxide, and N2 respectively. |
| Complete ionic equation | A balanced equation that describes a reaction occurring in solution, in which all strong electrolytes are written as dissociated ions. |
| Complexing agent | A ligand that binds to a metal ion to form a complex. |
| Complexometric titration | A titration based on a reaction between a ligand and a metal ion to form a complex. For example, free Ca2+ in milk powder can be determined by titrating a milk powder sample with EDTA solution, which chelates calcium ion. Endpoints in complexometric titrations are often determined using organochromic indicators. |
| Complex ion | An ion formed by combination of simpler ions or molecules; for example, Co2+ combines with six molecules of water to form the complex ion Co(H2O)62+. |
| Component | 1. A substance whose concentration must be specified to describe the state of a mixture in which reactions are occurring. 2. A substance present in a mixture in which no reactions occur. |
| Compound | A compound is a material formed from elements chemically combined in definite proportions by mass. For example, water is formed from chemically bound hydrogen and oxygen. Any pure water sample contains 2 g of hydrogen for every 16 g of oxygen. |
| Computer-assisted drug design | Using computational chemistry to discover, enhance, or study drugs and related biologically active molecules. |
| Computational chemistry | A branch of chemistry concerned with the prediction or simulation of chemical properties, structures, or processes using numerical techniques. |
| Concentrate | To increase the amount of substance present in a unit amount of mixture. For example, allowing solvent to evaporate from a solution concentrates the solution. |
| Concentrated | Having a relatively large amount of substance present in a unit amount of mixture. For example, a 12 M HCl solution is more concentrated than an 0.001 M HCl solution. |
| Concentration | 1. A measure of the amount of substance present in a unit amount of mixture. The amounts can be expressed as moles, masses, or volumes. 2. The process of increasing the amount of substance in a given amount of mixture. |
| Condensation | 1. The conversion of a gas into a liquid is called condensation. Condensation usually occurs when a gas is cooled below its boiling point. 2. A reaction that involves linking of two molecules with the elimination of water (or another small molecule). |
| Conformers | Molecular arrangements that differ only by rotations around single bonds. For example, the "boat" and "chair" forms of cyclohexane are conformers. |
| Congener | 1. Elements belonging to the same group on the periodic table. For example, sodium and potassium are congeners. 2. Compounds produced by identical synthesis reactions and procedures. |
| Constructive interference | When the peaks and troughs of two interfering waves match, the amplitudes add to give the resultant wave higher amplitude. |
| Continuous spectrum | A plot of the relative absorbance or intensity of emitted light vs. wavelength or frequency that shows a smooth variation, rather than a series of sharp peaks or bands. |
| Conversion factor | A conversion factor is a fraction that relates one unit to another. Multiplying a measurement by a conversion factor changes the units of the measurement. For example, since 1 in = 2.54 cm, to convert 10 inches to centimetres,
|
| Coordination number | The number of bonds formed by the central atom in a metal-ligand complex. |
| Copolymer | A polymer composed of two or more different monomers. The different monomers can be linked randomly, or in repeating sequences, or in blocks, or as side chains off the main chain. |
| Core electron | Electrons occupying completely filled shells under the valence shell. |
| Corrosion | Corrosion is a reaction that involves action of an oxidizing agent on a metal. The oxidizing agent is often oxygen dissolved in water. |
| Coulomb | (C) The SI unit of electric charge, equal to the amount of charge delivered by a current of 1 ampere running for 1 second. One mole of electrons has a charge of about 96487 C. |
| Coulombic interactions | electrostatic interactions. Attractions between opposite charges or repulsions between like charges that grow stronger as the charges become closer to each other. |
| Covalent bond | A covalent bond is a very strong attraction between two or more atoms that are sharing their electrons. In structural formulas, covalent bonds are represented by a line drawn between the symbols of the bonded atoms. |
| Covalent compound | A compound made of molecules- not ions. The atoms in the compound are bound together by shared electrons. Also called a molecular compound. |
| Critical point | State at which two phases of a substance first become indistinguishable. For example, at pressures higher than 217.6 atm and temperatures above 374°C, the meniscus between steam and liquid water will vanish; the two phases become indistinguishable and are referred to as a supercritical fluid. |
| Critical molar volume | (Vc) The molar volume at the critical point. |
| Critical pressure | (Pc) The pressure at the critical point. |
| Critical temperature | (Tc) The temperature at the critical point. A gas above the critical temperature will never condense into a liquid, no matter how much pressure is applied. Most substances have a critical temperature that is about 1.5 to 1.7 times the standard boiling point, in kelvin. |
| Cryogen | cryogenic gas. A gas that has been liquified by lowering temperature, usually to a temperature under about -100°C. |
| Crystal | A sample of a crystalline solid that has a regular shape bound by plane surfaces (facets) that intersect at characteristic angles. The shape results from the arrangement of the substances atoms, ions, or molecules. Most crystals contain defects that can strongly affect their optical and electrical properties. |
| Crystallite | A perfect crystalline part of a larger imperfect crystal. Real crystals are usually built of a large number of crystallites. |
| Crystallization | Production of a purer sample of a substance by slow precipitation of crystals from a solution of the substance. |
| Crystal field splitting energy | ( Ligands complexed to a metal ion will raise the energy of some of its d orbitals and lower the energy of others. The difference in energy is called the crystal field splitting energy. |
| Crystal field theory | The colour, spectra, and magnetic properties of metal-ligand complexes can be explained by modelling the effect of ligands on metal's d orbital energies. |
| Crystalline solid | A solid that has a repeating, regular three-dimensional arrangement of atoms, molecules, or ions. |
| Crystallization | The process of forming pure crystals by freezing a liquid, evaporating a solution, or precipitating a solid from solution. Impurities remain in the liquid, so crystallization is often to purify solid substances. |
| Cupric | Cu2+) cupric ion. Deprecated. 1. the copper(II) ion, Cu2+. 2. A compound that contains copper in the +2 oxidation state. |
| Cuprous | (Cu+) cuprous ion. Deprecated. 1. the copper(I) ion, Cu+. 2. A compound that contains copper in the +1 oxidation state. |
| Curie point | Temperature above which a ferromagnetic material loses its ferromagnetism. |
| Cyanide | (CN-) 1. An ion with a -1 charge containing one atom of carbon bound to one atom of nitrogen. 2. A compound that contains CN- ions. |
| Cyanide process | A method for separating a metal from an ore. Crushed ore is treated with cyanide ion to produce a soluble metal cyanide complex. The complex is washed out of the ore and reduced to metallic form using an active metal (usually zinc). |
| Cysteine | 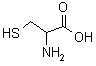 |
| Cystine | 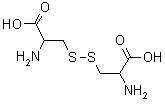 |
Document Actions

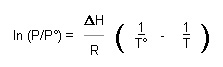
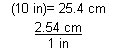
 Like us on Facebook
Like us on Facebook