Bonding
Why Atoms Join Together
Compounds are formed when two or more different elements are chemically bonded together.
The elements in a compound are bonded together very strongly, so are difficult to separate.
Atoms bond together in order to gain a stable electron structure, similar to the noble gases.
The noble gases are very unreactive and don't form compounds because their atoms have full outer shells of electrons.
Atoms achieve a full outer shell of electrons by either gaining or losing electrons, as in ionic bonding, or sharing electrons, as in covalent bonding.
Ionic Bonding
The atoms gain or lose electrons to form charged particles called ions.
Atoms which lose electrons form positively charged ions, atoms which gain electrons form negatively charged ions.
Sodium, 2311Na has eleven electrons. Its electron arrangement is 2, 8, 1.
When sodium reacts with other elements, its atoms lose the outer electron to form a positively charged ion.

A chlorine atom has 17 electrons. Its electron arrangement is 2, 8, 7. Chlorine gains one electron to fill its outer shell and form a negatively charged ion.

In an ionic bond, oppositely charged ions are strongly held together by electrostatic forces.
Sodium and chloride ions bond together to form sodium chloride, NaCl.
No charges are shown in the formula because they are cancelled out.
You should also be able to represent the ions in magnesium oxide, MgO, and calcium chloride, CaCl2.
Ionic compounds form regular structures called giant ionic lattices. They have high melting and boiling points because there are many strong bonds to overcome between the ions.
When melted or dissolved in water, ionic compounds conduct electricity because the ions are free to move.
Covalent Bonding
The atoms achieve a stable electron structure by sharing pairs of electrons in their outer shells.
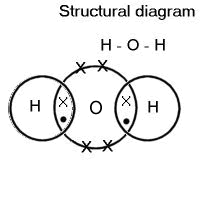
Many covalent compounds are simple molecules, such as water, H2O.
Hydrogen has only one electron in the first shell. This shell can hold two electrons, so it needs another electron to fill it.
Oxygen has six electrons in its outer shell, so needs two more to fill it.
The oxygen forms a shared pair of electrons with each hydrogen atom; so all atoms have full outer shells.
A shared pair of electrons is a covalent bond. One electron of the pair is from one atom and the other is from a different atom.
Covalent bonds are drawn like this, showing the outer shells of electrons only.
The dots and crosses represent the outer electrons of the different atoms.
In the structural diagram each line represents a shared pair of electrons – one covalent bond.
You should also be able to represent the covalent bonds in ammonia, NH3, hydrogen, H2, hydrogen chloride, HCl, methane, CH4 and oxygen, O2.
Simple molecular compounds have low melting and boiling points because the forces between the molecules are weak, although the covalent bonds within the molecules are strong.
They do not conduct electricity because the molecules do not have an electric charge.
Diamond, graphite and silicon dioxide (silica) are giant covalent structures of atoms. They have very high melting points because there are many covalent bonds in their structures.
Diamond and graphite are both forms of carbon.
In diamond, each carbon atom is covalently bonded to four other carbon atoms. The resulting rigid 3-D giant covalent structure is very strong. A diamond crystal is one giant molecule.
Silicon dioxide has a similar structure.
In graphite each carbon atom forms three covalent bonds, forming giant molecules in layers.
The bonds between the layers are weak, so it is easy for the layers to slide over each other.
Graphite contains free electrons, so it can conduct electricity.

Metallic Bonding
Metals are giant structures.
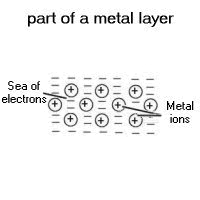
The outer electrons of the metal atoms are free to move through the whole structure. This forms tightly packed layers of ions, surrounded by a ‘sea' of electrons.
The charges on the ions and electrons hold the structure together, but allow the ions to slide over each other. This is why metals can be bent and stretched.
The free electrons allow the metal to conduct electricity and heat.
Document Actions

 Like us on Facebook
Like us on Facebook