Rates of Reactions
Measuring the rate of a reaction
Different reactions proceed at different speeds.
Rusting is a very slow reaction, whereas an explosion is a very fast reaction.
A chemical reaction can be followed by measuring the rate at which a product is formed, or the rate at which the reactants are used up.
When sodium thiosulphate solution is reacted with hydrochloric acid, one of the products is sulphur. This appears as a fine yellow precipitate, which makes the liquid go cloudy.
The rate of this reaction can be measured by timing how long it takes for enough sulphur to form so that a dark cross drawn on a piece of card below the reaction flask can no longer be seen.
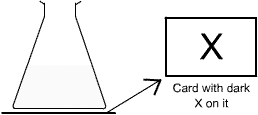
This method measures the end point of the reaction.
If a gas is being formed the volume produced can be measured at regular time intervals using a gas syringe, so the rate throughout a reaction can be followed.
A graph of the results can then be plotted.
e.g. magnesium + hydrochloric acid ![]() magnesium chloride + hydrogen
magnesium chloride + hydrogen
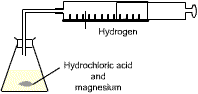
The rate is fastest at the start because there are more reacting particles present.

The rate for the first minute is 14 cm3 per minute.
The faster the reaction, the steeper the curve.
When the reaction is over the curve is flat. No more gas is produced because one or both of the reactants has been used up.
A similar method is to measure the loss of mass as a gas escapes from a reaction vessel at regular time intervals. A graph of loss in mass against time can be plotted.
Factors affecting the rate of a reaction
In order for a chemical reaction to occur the particles must:
- collide with each other
and - have sufficient energy to react
The minimum amount of energy the particles must have is called the activation energy.
The rate depends on the number of successful collisions there are in a given time.
The rate of a chemical reaction increases if:
- the temperature increases. Increasing the temperature increases the speed of the particles so they collide more frequently. They also have more energy so more collisions are successful.
- the concentration of dissolved reactants or the pressure of gases increases. There are more particles in a given volume, so they are more likely to collide with each other.
- the solid reactants are broken into smaller pieces. This increases their surface area, so there are more places exposed for particles to collide with.
- a catalyst is used. A catalyst is a substance which speeds up a chemical reaction but is not used up during the reaction. It can be used over and over again. Different reactions need different catalysts.
Increasing the rates of chemical reactions is important in industry because it helps to reduce costs.
A hot iron catalyst is used in the Haber process to produce ammonia.
A hot platinum catalyst is used in the manufacture of nitric acid.
Comparing rates
The graph below shows the results of an experiment to investigate the effect of surface area on the rate of reaction.
Large pieces of calcium carbonate (marble chips) were reacted with hydrochloric acid and the loss in mass was measured every minute as carbon dioxide gas was given off.
calcium carbonate + hydrochloric acid ![]() calcium chloride + carbon dioxide + water
calcium chloride + carbon dioxide + water
The reaction was then repeated using the same volume and concentration of acid and the same mass of marble chips.
The only difference was that the chips were smaller, so had a bigger surface area.

The smaller pieces reacted faster, as shown by the steeper curve.
This reaction therefore finished sooner than with large pieces.
Both reactions lost the same mass, 2 g of carbon dioxide, so leveled off at the same position. This is because there was the same amount of reactants in both experiments.
Document Actions

 Like us on Facebook
Like us on Facebook